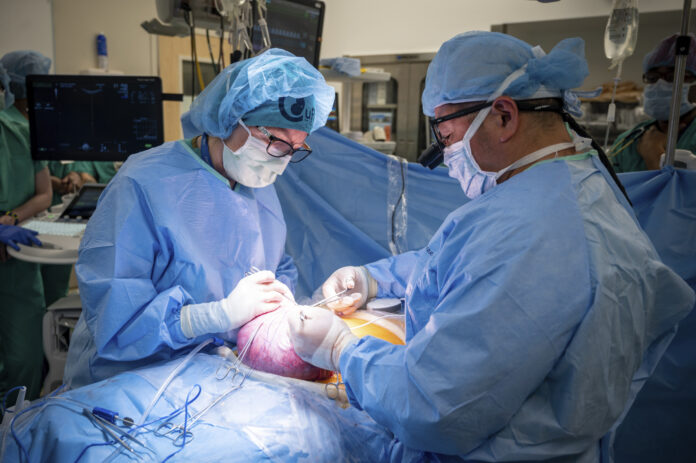
The procedure marks the first time paralysis has been successfully cured in utero
By BRANDON NGUYEN — science@theaggie.org
For the first time in the world, UC Davis Health successfully cured paralysis and predicted associated neurological defects with spina bifida for babies in utero during fetal surgery.
According to UC Davis Health’s website, spina bifida, also known as myelomeningocele, is not uncommon, affecting 1,500 to 2,000 children in the U.S. annually. This neurological condition is diagnosed through a blood test and ultrasound screenings when spinal tissue fails to fuse properly during the early stages of pregnancy, which can lead to a multitude of lifelong disabilities related to cognitive function, mobility and urinary and bowel movements.
Until now, fetal surgery alone did not guarantee recovery in these patients, but with the rise of stem cell technology, hope for a cure is much closer to reality. Dr. Diana Lee Farmer, the chair for the Department of Surgery as well as a trained fetal surgeon at UC Davis Health, launched the landmark CuRe Trial, which stands for Cellular Therapy for In Utero Repair of Myelomeningocele, in spring 2021 to begin recruiting patients to test their placental stem cells during fetal surgery.
Farmer has dedicated over 20 years to searching for a cure for this condition, but she explained that regenerative stem cell therapy only recently became a feasible solution.
“For Spina bifida, kids don’t die, but it’s terrible,” Farmer said. “They’re born paralyzed for the rest of their life, and there’s really nothing anybody could do at the time. They have fluid on their brains; they can’t pee or poop. They have to have special devices to help with urinary and bowel movements. It’s not fatal, but it’s devastating to both patients and families and the cost to society.”
According to Farmer, the stem cells her team extracted came from the placenta, which she said is an organ that is rich in cells undergoing constant regeneration but is often thrown out following a baby’s delivery.
“We discovered that these unique placenta-derived stem cells had special characteristics,” Farmer said. “They sort of secrete what I call ‘magic stem cell juice.’ In addition to protecting the spinal cord — at least in our animal models and in how our bulldogs respond to it — it looks like it reversed some of the damage that was already done, because we had complete recovery in those experimental models. So we worked hard to get donor placentas and extract the cells and engineer them […] and eventually got approval from the FDA to do the first human studies to see if this was going to be safe in humans.”
Dr. Aijun Wang, a trained bioengineer and an associate professor of the Department of Surgery for the UC Davis School of Medicine, helped Farmer develop the stem cell patch to be used in the surgery. Preliminary work by the two showed that prenatal surgery combined with the human-placenta-derived stem cells, held in place with a biomaterial scaffold to form a “patch,” helped lambs with spina bifida walk without noticeable disability.
“When the baby sheep who received stem cells were born, they were able to stand at birth and they were able to run around almost normally; it was amazing,” Wang said.
The team also altered the technique used on lambs that combined surgery with stem cells for canines. When both the models on lambs and canines restored the animals’ ability to walk, Farmer and her team moved to begin a human trial.
One patient who took part in the CuRe trial was Michelle Johnson, who flew down with her husband Jeff Maginnis from Portland, Oregon to receive the placental stem cells during fetal surgery. Johnson detailed the rollercoaster of emotions she felt upon hearing the diagnosis.
“I remember that around the twenty-week mark in the gestation period, I received a call from my radiologist following my ultrasound,” Johnson said. “His voice was trembling, and he had told me that my baby had a spinal defect called spina bifida. I got off the phone, thinking, ‘what is spina bifida?’ It’s not genetic; it’s very random in presentation. I had no control over this, so why me and why my child?”
Johnson said that the diagnosis left her feeling like she had no options, but the CuRe trial at the time became the miracle she needed.
“I was willing to do anything to give our baby the highest quality of life possible, and that’s when I heard about the CuRe trial,” Johnson said. “We could’ve lost our careers in risking a lot when we moved down to Davis, but it was worth giving our son the ability to walk. Tobi is a little miracle; he’s about to crawl and is the happiest baby in the world.”
For Farmer, reversing spina bifida’s effects has paved the way for reaching a feasible cure for other neurological diseases.
“We’re starting to work with members of the team on a variety of other neurodegenerative or neural diseases that might be related to inflammation,” Farmer said. “The magic stem cell juice is really magic. If you think about it, the placenta is a very interesting organ. It goes from nothing to being done and fully formed and dying essentially in nine months. The organ makes big blood vessels and protects the fetus from being rejected. So I think there’s going to be more potential on placental research as well as further stem cell applications.”
Written by: Brandon Nguyen — science@theaggie.org